Quantum Mechanical Model of Atom – Schrödinger Equation
Quantum Mechanical Model of Atom – Schrödinger Equation: Overview
This Topic covers sub-topics such as Shapes of Orbitals, Schrodinger's Wave Equation, Quantum Mechanical Model of Atom, Angular Nodes, Radial Nodes, Radial Probability Density, Radial Probability and, Characteristics of Orbitals
Important Questions on Quantum Mechanical Model of Atom – Schrödinger Equation
The number of spherical nodes in 3p orbitals are
For radial probability curves, which of the following is/are correct?
Plot of radial wave function as a function of distance of the electron from the nucleus for orbital.
The number of radial nodes in and orbitals are respectively:
The number of nodal planes in orbital is _____.
The number of nodal planes in orbital is
The region of space around the nucleus where the probability of finding an electron is maximum is called_____.
What is the quantum mechanical model of an atom?
_____ developed a quantum mechanical model of an atom.(Rutherford/Erwin Schrodinger)
Do the electrons follow the definite paths around the nucleus?
The number of nodal planes ‘ ’ orbital has, is:
| Column I (orbital) | Column II (Radial nodes) | ||
| A | 3p | p | 0 |
| B | 5d | q | 1 |
| C | 4f | r | 2 |
| D | 4s | s | 3 |
(Assume that all graphs have been plotted for the same parameters. In all these curves n )
Choose the correct radial probability curve for orbital.
Number of radial nodes for orbital
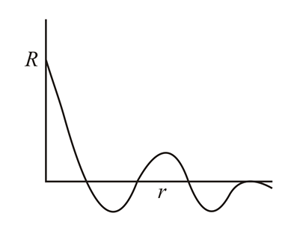
From the following graph between R (radial wave function) and r (distance from nucleus), identify orbital:-
is Bohr's radius of the first orbit. In the light of the wave mechanical model, it suggests that
Choose the correct options:
The radial distribution function is used to determine the most probable radius which is used to find the electron in a given orbital. The is given by . Then, which of the following statements are correct?
Mr. Santa has to decode a number "ABCDEF" where each alphabet is represented by a single digit. Suppose an orbital whose radial wave function is represented as $\Psi_{(r)}=k_{1} e^{-r / k_{2}}\left(r^{2}-5 k_{3} r+6 k_{3}^{2}\right)$
From the following information given about each alphabet then write down the value of $\{(A \times D)+(B+C+E+F)\}$ where each alphabet corresponds to a value.
Info $\mathrm{A}=$ Value of $\mathrm{n}$ where "n" is principal quantum number
Info $\mathrm{B}=\mathrm{No}$. of angular nodes
Info $\mathrm{C}=$ Azimuthal quantum number of subshell to which orbital belongs Info $\mathrm{D}=$ No. of subshells having energy between $(\mathrm{n}+5) \mathrm{s}$ to $(\mathrm{n}+5) \mathrm{p}$ where $\mathrm{n}$ is principal quantum number
Info $E=$ Orbital angular momentum of given orbital.
Info $\mathrm{F}=$ Radial distance of the spherical node which is farthest from the nucleus (Assuming: $\mathrm{k}_{3}=1$ )
The ion is a hydrogen like ion that can be described by the Bohr model. Calculate the Bohr radius of the third orbit and calculate the energy of an electron in th orbit.
